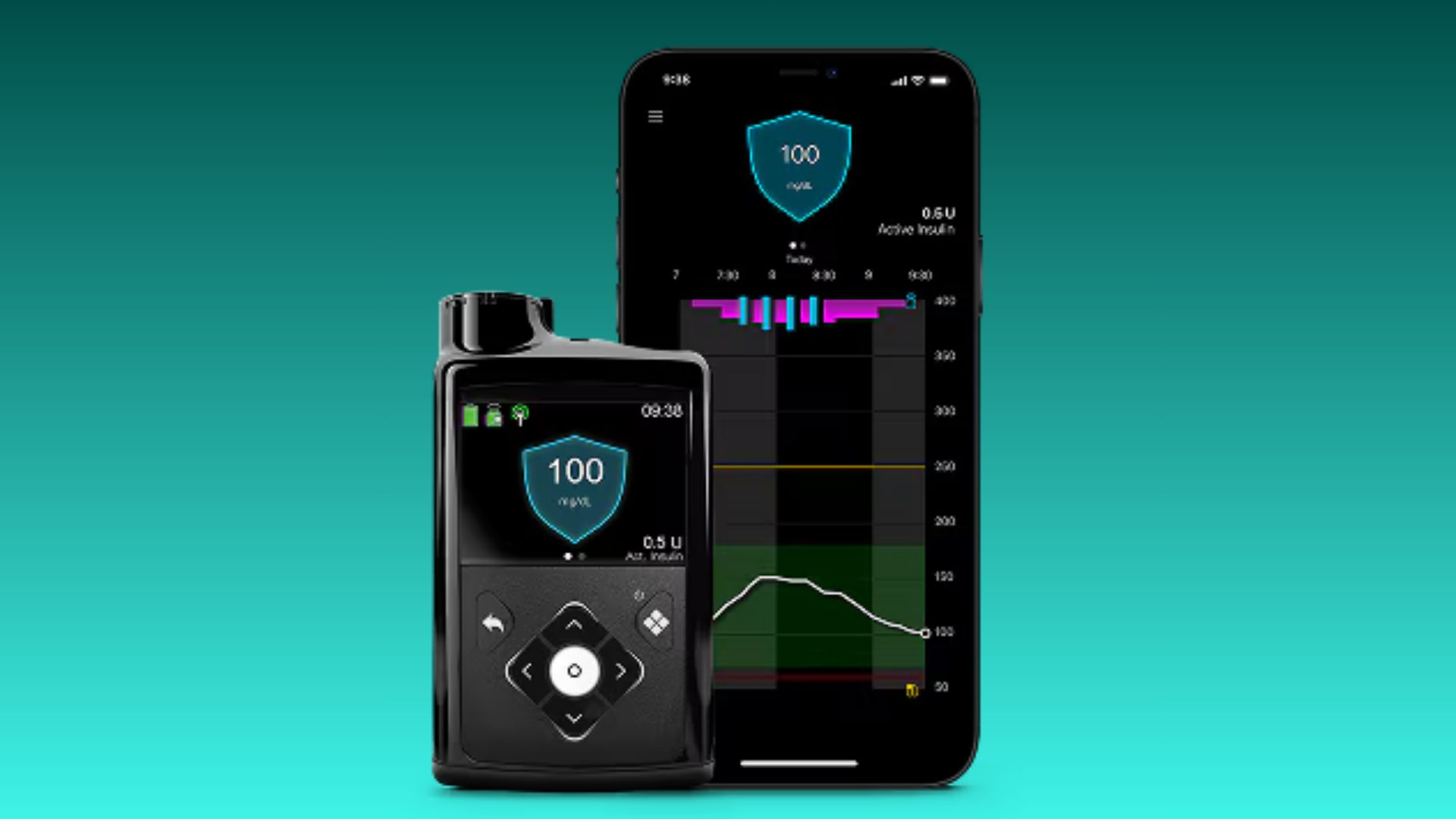
Medtronic has announced a series of regulatory and access milestones. The company received FDA clearance for its MiniMed 780G insulin pump to be used with ultra rapid-acting insulins Fiasp and Lyumjev. This update allows insulin delivery to more closely mirror the body’s natural response around meals, supporting tighter glucose control and greater flexibility for users.
In addition, Medtronic secured Medicare coverage for the MiniMed 780G when used with the Instinct continuous glucose monitor (CGM) made by Abbott, broadening access for older adults and others covered under Medicare. The FDA also cleared the MiniMed 780G and Instinct sensor combination for people with insulin-requiring type 2 diabetes, expanding the system’s indicated population beyond type 1 diabetes.
Looking ahead, Medtronic is continuing to invest in product innovation with the development of two new insulin pumps: the MiniMed Flex, a tubed pump, and the MiniMed Fit, a patch pump.
Want more?
For the latest diabetes tech, join our free newsletter.
If you like our content and want more, join Diabetech All Access—unlocking monthly Live Q&As, exclusive stories and industry analysis. Your support helps sustain our independent journalism and keeps this platform thriving.
Disclaimer: Diabetech content is not medical advice—it’s for educational purposes only. Always consult with a physician before making changes to your healthcare.